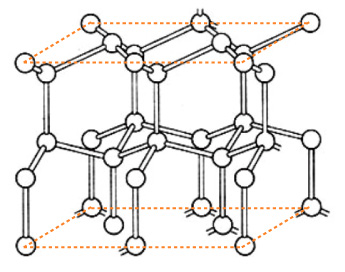
氷の通常の結晶 (「Ih」) は,各水分子の酸素原子が<隣接の4個の酸素原子が張る正四面体>の中心に位置する。
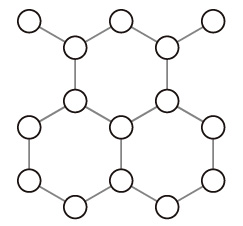
 上図の破線枠で表した面に対し垂直に見ると:
上図の破線枠で表した面に対し垂直に見ると:
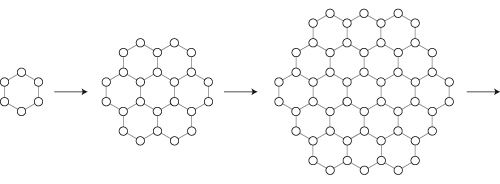
ここで,上下二底面を維持しつつ水分子が付着していくことを考える。
等方的でかつ側面をつくる形は,正六角柱になる:
 Libbrecht : Snow Crystal Science から引用:
Libbrecht : Snow Crystal Science から引用:

水分子付着のメカニズムは:
| |
Libbrecht : Attachment Kinetics
Several molecular processes occurring on the surface of a growing crystal :
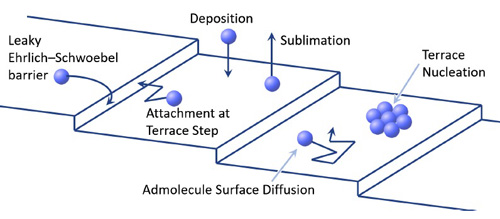
If lattice dislocations are present on a faceted surface, then growth can sometimes proceed without the need to nucleate new molecular terraces. The most common mechanism for dislocation-mediated facet growth is via screw dislocations.
The lattice structure of a screw dislocation :
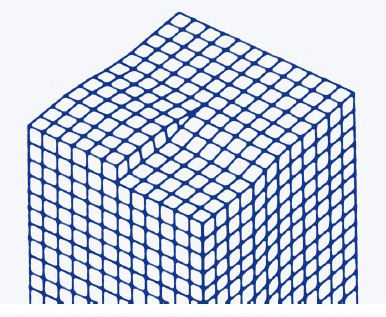
|
As the dislocation edge grows from admolecule attachment, it creates a spiral pattern that can propagate indefinitely, yielding growth without terrace nucleation :
|
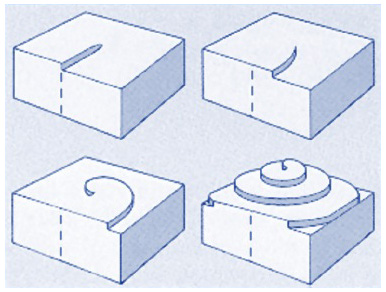
|
|
以下,雪結晶の生成過程:
| |
Libbrecht : Snowflake Science
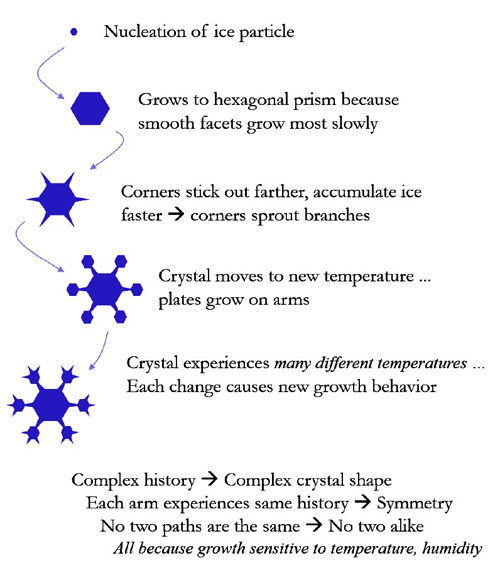
A snow crystal appears when water vapor in the air converts directly into ice without first becoming liquid water.
As more water vapor condenses onto a nascent snow crystal, it grow and develops, and that is when its ornate patterns emerge.
‥‥
The exact shape of the final snow crystal is determined by the precise path it took through the clouds.
But the six arms all took the same path, and so each experienced the same changes at the same times.
Thus the six arms grow in synchrony, yielding a complex, yet symmetrical shape.
And since no two snow crystals follow the exact same path through the clouds as they fall, no two look exactly alike.
‥‥
The six-fold symmetry you see in a snow crystal arises from the arrangement of water molecules in the ice crystal lattice.
‥‥
When snow crystals first begin growing, they are shaped like the simple hexagonal prisms ‥‥.
Each prism has two basal facets and six prism facets.
Hexagonal prisms display the simple, perfect order of the molecular lattice.
These crystals result from slow growth, and they are usually small in size.
‥‥
The chaotic branching of a fernlike stellar dendrite arises during rapid ice growth, in contrast to the slow, simple growth of hexagonal prisms.
‥‥
The different growth processes guide snow crystal growth differently.
Faceting creates order, as embodied by the simple, perfect, hexagonal prism.
Branching brings chaos, as embodied by the randomly spaced sidebranches in a fernlike stellar dendrite.
|
|
| |
Libbrecht : Snow Crystal Science
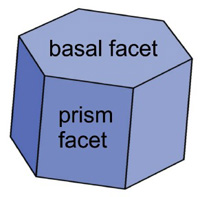 The most basic shape of a snow crystal is a hexagonal prism with two basal facets and six prism facets.
The most basic shape of a snow crystal is a hexagonal prism with two basal facets and six prism facets.
This shape arises because of the underlying hexagonal structure of the ice crystal lattice.
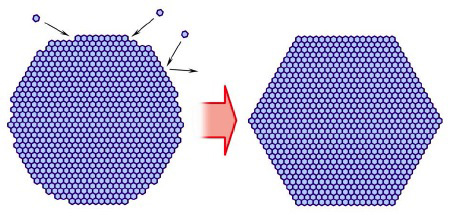 When water vapor molecules strike a molecularly rough ice surface, they tend to stick.
When water vapor molecules strike a molecularly rough ice surface, they tend to stick.
But when they strike a molecularly smooth facet surface, they are less likely to stick.
As the crystal grows, soon the rough areas fill in, leaving a fully faceted ice prism.
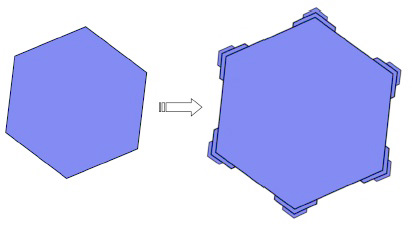 The six corners of a thin hexagonal plate stick out into the humid air around it.
The six corners of a thin hexagonal plate stick out into the humid air around it.
Water vapor condenses preferentially on the corners as a result, making them stick out even farther.
This leads to a branching instability that causes six branches to sprout from the corners of the hexagon.
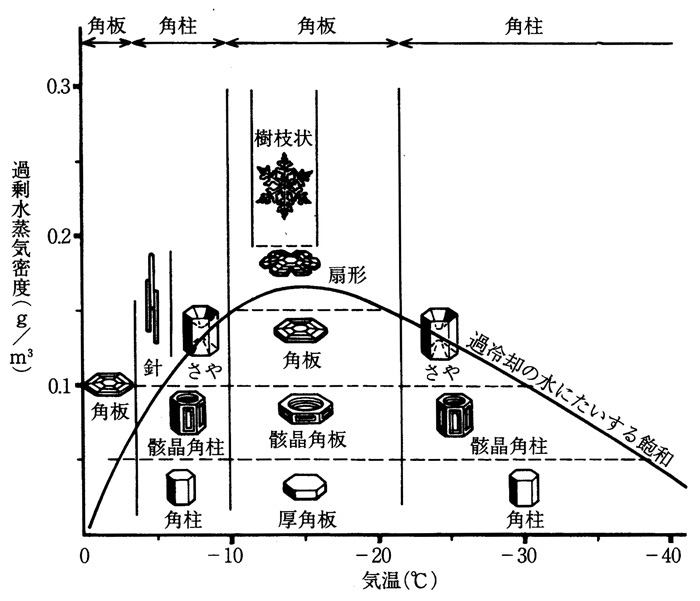
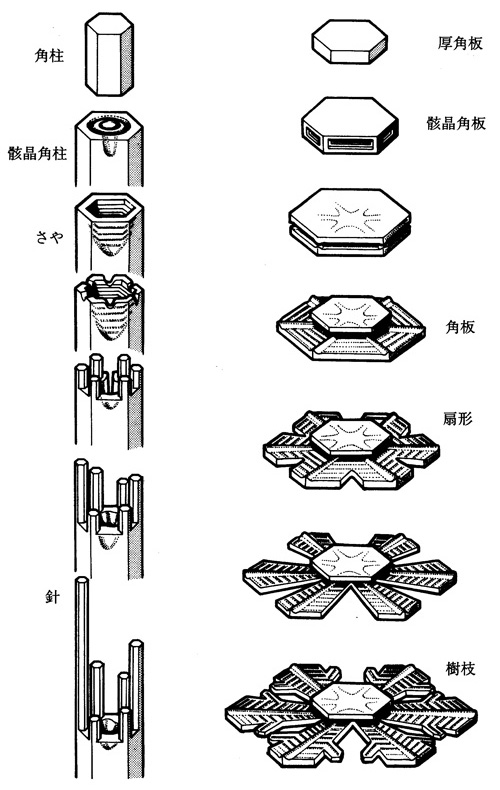
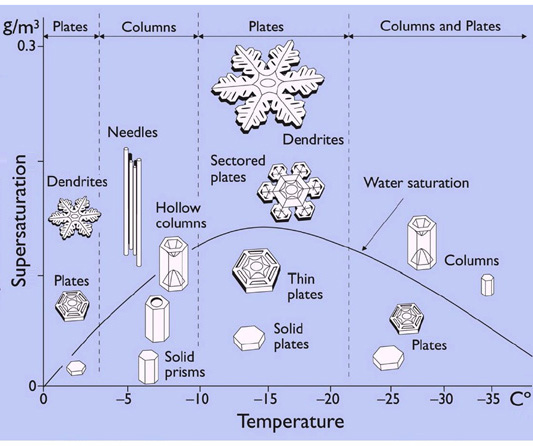 This snow-crystal morphology diagram illustrates the types of snow crystals that develop at different temperatures and supersaturations.
This snow-crystal morphology diagram illustrates the types of snow crystals that develop at different temperatures and supersaturations.
The water saturation line shows the supersaturation that is typically found in a dense winter cloud made of liquid water droplets.
|
|
|